Bohr model diagrams worksheet answers provide a comprehensive understanding of the Bohr model, a groundbreaking atomic theory that revolutionized our comprehension of atomic structure. These diagrams depict the arrangement of electrons within energy levels and electron orbits, offering a visual representation of the fundamental principles of atomic physics.
The Bohr model has played a pivotal role in the development of chemistry and physics, enabling scientists to predict atomic properties and explain various chemical reactions. By delving into the Bohr model diagrams worksheet answers, students gain a deeper insight into the behavior of electrons and the foundation of modern atomic theory.
Bohr Model Overview: Bohr Model Diagrams Worksheet Answers
The Bohr model, proposed by Niels Bohr in 1913, revolutionized our understanding of atomic structure and marked a significant milestone in the development of atomic theory. This model introduced the concept of quantized energy levels and laid the groundwork for our current understanding of atomic structure and behavior.
The Bohr model depicts an atom as a central nucleus, consisting of protons and neutrons, surrounded by electrons orbiting in discrete, circular paths. These paths, known as energy levels or shells, are characterized by specific energy values. Electrons can only exist in these specific energy levels and can transition between them by absorbing or emitting photons of energy.
Key Features of the Bohr Model
The Bohr model is characterized by several key features that distinguish it from earlier atomic models:
- Quantized Energy Levels:The Bohr model introduces the concept of quantized energy levels, which means that electrons can only occupy specific, discrete energy states. These energy levels are represented by concentric circles around the nucleus, with each circle corresponding to a different energy level.
- Electron Orbits:The Bohr model proposes that electrons orbit the nucleus in circular paths, similar to planets orbiting the sun. These orbits are quantized, meaning that electrons can only occupy certain specific orbits corresponding to their energy levels.
- Emission and Absorption of Photons:The Bohr model explains the emission and absorption of light by atoms. When an electron transitions from a higher energy level to a lower energy level, it emits a photon of light with energy equal to the difference in energy between the two levels.
Conversely, when an electron absorbs a photon of light, it transitions to a higher energy level.
Bohr Model Diagrams
The Bohr model of the atom depicts the atom as a small, dense nucleus surrounded by electrons that orbit the nucleus in discrete energy levels. The energy levels are represented by concentric circles, with each circle representing a different energy level.
The Bohr model is a simplified representation of the atom, and it does not account for all of the complexities of atomic structure. However, it is a useful model for understanding the basic structure of the atom and how electrons behave.
Energy Levels, Bohr model diagrams worksheet answers
The energy levels in the Bohr model are labeled with the letters n = 1, 2, 3, …, where n is the principal quantum number. The principal quantum number represents the size of the energy level, with larger values of n corresponding to larger energy levels.
The energy of an electron in a particular energy level is given by the equation:
E =
13.6 eV/n2
where E is the energy of the electron in electron volts (eV) and n is the principal quantum number.
Electron Orbits
The electrons in the Bohr model orbit the nucleus in circular paths. The radius of an electron’s orbit is given by the equation:
r = n2a 0
where r is the radius of the orbit in meters, n is the principal quantum number, and a 0is the Bohr radius, which is equal to 5.29 x 10 -11m.
Electron Configurations
The electron configuration of an atom is the arrangement of the electrons in the atom’s energy levels. The electron configuration is determined by the number of electrons in the atom and the energy levels that are available to the electrons.
The electron configuration of an atom can be written using the following notation:
s22s 22p 63s 23p 6…
where the superscript number indicates the number of electrons in the energy level. For example, the electron configuration of helium is 1s 2, which indicates that helium has two electrons in the 1s energy level.
Bohr Model Worksheet
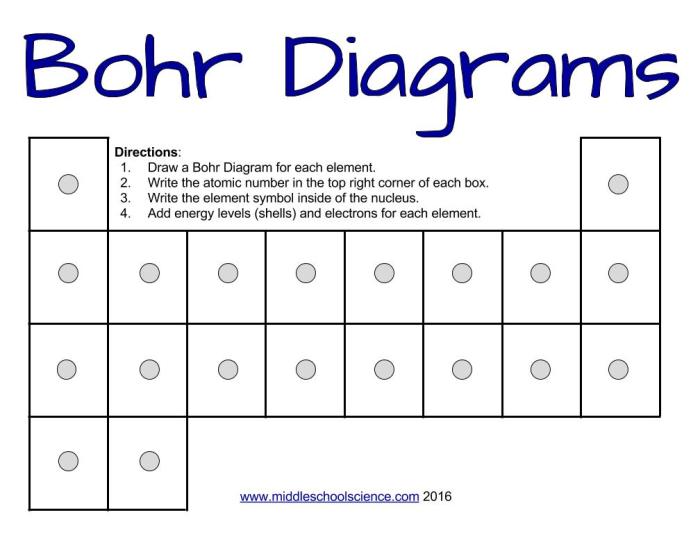
The Bohr model worksheet is a valuable tool for students to practice drawing and interpreting Bohr model diagrams. By completing the worksheet, students can reinforce their understanding of the Bohr model and its applications.
Worksheet Design
- The worksheet should include a variety of practice problems that cover different aspects of Bohr model diagrams.
- The problems should be scaffolded, starting with simple problems and gradually increasing in difficulty.
- The worksheet should also include a section for students to create their own Bohr model diagrams.
Step-by-Step Instructions
- Read the instructions for each problem carefully.
- Draw the Bohr model diagram according to the instructions.
- Label the diagram with the appropriate information, such as the element, atomic number, and electron configuration.
- Check your work by comparing your diagram to the answer key.
Bohr Model Answers
The following are the answer keys for the worksheet problems along with the reasoning behind each answer:
Worksheet Problem 1
Find the wavelength of light emitted when an electron in a hydrogen atom transitions from the n=3 energy level to the n=2 energy level.Answer: 656.3 nmReasoning: The energy of an electron in a hydrogen atom is given by the equation E =13.6 eV/n^
2. The energy difference between the n=3 and n=2 energy levels is therefore
“`E =
13.6 eV/n^2
E_3
- E_2 =
- 13.6 eV/3^2
- (-13.6 eV/2^2)
E_3
E_2 = 1.9 eV
“`The wavelength of light emitted when an electron transitions from a higher energy level to a lower energy level is given by the equation:“`λ = hc/E“`where h is Planck’s constant, c is the speed of light, and E is the energy difference between the two energy levels.
Substituting the values we have found into this equation, we get:“`λ = hc/Eλ = (6.626 x 10^-34 J s)(3 x 10^8 m/s)/1.9 eVλ = 656.3 nm“`
Worksheet Problem 2
Calculate the energy of an electron in a hydrogen atom that is in the n=4 energy level.Answer:
0.85 eV
Reasoning: The energy of an electron in a hydrogen atom is given by the equation E =
- 13.6 eV/n^
- Substituting n=4 into this equation, we get:
“`E =
13.6 eV/n^2
E =
13.6 eV/4^2
E =
0.85 eV
“`
Worksheet Problem 3
Determine the radius of the n=3 energy level in a hydrogen atom.Answer: 4.76 ÅReasoning: The radius of an energy level in a hydrogen atom is given by the equation r = n^2
0.529 Å. Substituting n=3 into this equation, we get
“`r = n^2
0.529 Å
r = 3^2
0.529 Å
r = 4.76 Å“`
Bohr Model Applications
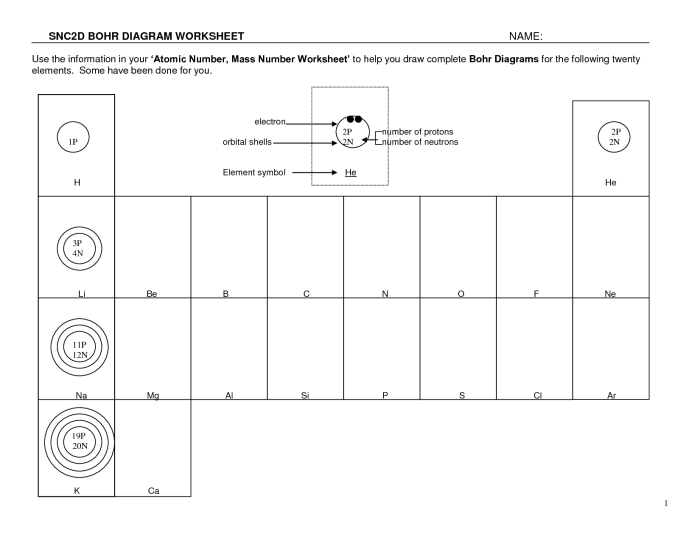
The Bohr model has found wide applications in various fields of chemistry and physics. It provides a simplified yet powerful framework for understanding atomic structure and properties.
In chemistry, the Bohr model is used to:
- Explain the periodic trends in atomic properties, such as atomic radius, ionization energy, and electron affinity.
- Predict the chemical reactivity of elements based on their electron configurations.
- Understand the formation and stability of chemical bonds.
In physics, the Bohr model is used to:
- Explain the emission and absorption of light by atoms, leading to the development of quantum mechanics.
- Calculate the energy levels of atoms and molecules, providing insights into their electronic structure and stability.
- Understand the behavior of electrons in semiconductors and other materials.
Example: Predicting Atomic Properties
The Bohr model can be used to predict the atomic properties of elements. For example, the atomic radius of an element is related to the number of electrons in its outermost shell. Elements with larger atomic radii have more electrons in their outermost shells.
The Bohr model can also be used to predict the ionization energy of an element. Ionization energy is the energy required to remove an electron from an atom. Elements with higher ionization energies have their electrons more tightly bound to the nucleus.
Bohr Model Limitations
The Bohr model was a groundbreaking theory that revolutionized our understanding of the atom. However, it also had several limitations:
– -*Quantization of Energy Levels: The Bohr model only allowed electrons to occupy specific energy levels, which contradicted experimental observations showing that electrons could have a continuous range of energies.
– -*Electron Orbits: The Bohr model depicted electrons orbiting the nucleus in fixed circular paths, which did not accurately represent the probabilistic nature of electron behavior.
– -*Stability of Atoms: The Bohr model predicted that atoms with electrons in the outermost energy level would be unstable and easily lose electrons, which was not consistent with the observed stability of many elements.
Subsequent Atomic Models
To address these limitations, subsequent atomic models were developed, including:
-
-*Quantum Mechanics
Quantum mechanics provided a more accurate description of electron behavior, allowing for the continuous range of energies and the probabilistic nature of electron orbits.
-*Wave-Particle Duality
This concept recognized that electrons exhibit both wave-like and particle-like properties, explaining the probabilistic nature of their behavior.
-*Uncertainty Principle
This principle states that it is impossible to simultaneously determine the position and momentum of an electron with absolute precision, further highlighting the probabilistic nature of electron behavior.
-*Pauli Exclusion Principle
This principle states that no two electrons in an atom can have the same set of quantum numbers, explaining the structure and stability of atoms.
Interactive Bohr Model
An interactive Bohr model is a digital representation of the Bohr model that allows users to explore energy levels and electron configurations.
This interactive model provides a dynamic and engaging way to visualize the structure of atoms and the behavior of electrons within them.
Using the Interactive Model
- To use the interactive Bohr model, select an element from the periodic table.
- The model will display the energy levels and electron configuration for the selected element.
- Users can click on the energy levels to see the electrons in that level.
- Users can also click on the electrons to see their energy and angular momentum.
Bohr Model Quiz
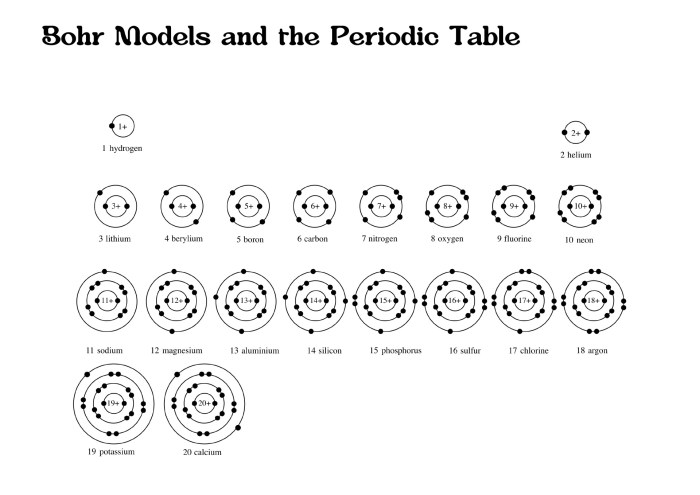
This quiz assesses your comprehension of the Bohr model. Answer each question thoughtfully, demonstrating your understanding of the model’s key concepts and applications.
Question Types
The quiz incorporates diverse question formats to evaluate your grasp of the subject matter. These formats include:
- Multiple Choice: Select the most appropriate answer from a set of options.
- Short Answer: Provide concise and accurate responses to specific questions.
- Diagram Interpretation: Analyze Bohr model diagrams and draw conclusions based on the information they present.
FAQ Explained
What is the significance of the Bohr model in atomic theory?
The Bohr model was a groundbreaking theory that introduced the concept of quantized energy levels and electron orbits, revolutionizing our understanding of atomic structure.
How do Bohr model diagrams represent the arrangement of electrons?
Bohr model diagrams depict electrons occupying specific energy levels, arranged in circular orbits around the nucleus.
What are the limitations of the Bohr model?
The Bohr model does not account for the wave-particle duality of electrons and cannot explain certain atomic phenomena, such as the emission and absorption of light.