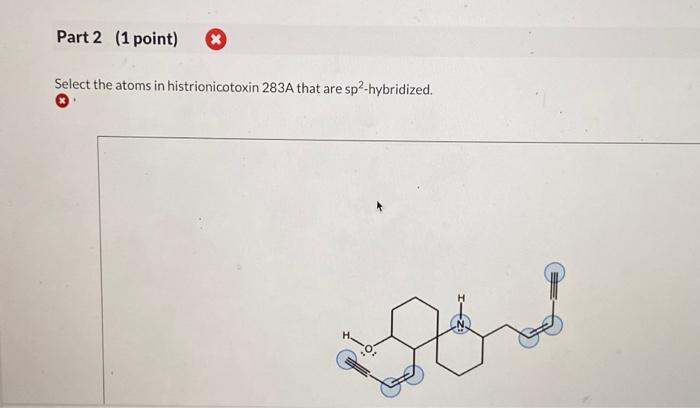
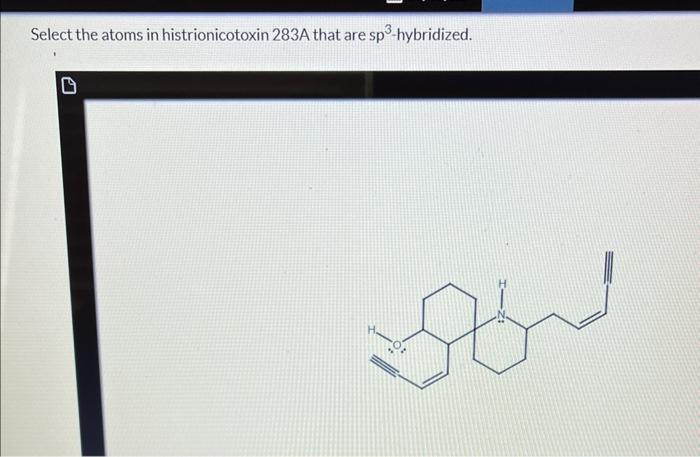
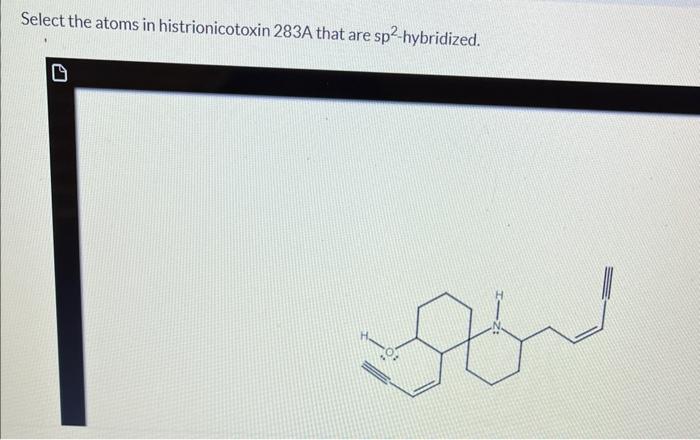
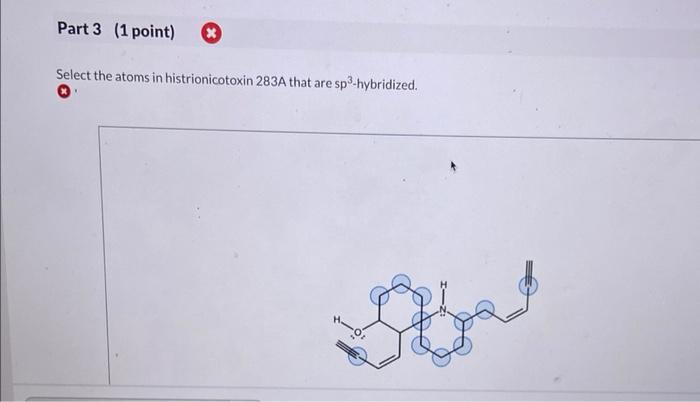
Select the atoms in histrionicotoxin 283a that are sp2-hybridized. – Embarking on a journey into the realm of sp2-hybridization, we delve into the intricacies of histrionicotoxin 283a, a molecule renowned for its biological significance. This exploration will uncover the pivotal role of sp2-hybridized carbon atoms in shaping the structure and function of this fascinating compound.
As we navigate the molecular landscape of histrionicotoxin 283a, we will unravel the unique properties of sp2-hybridization, examining its impact on geometry, bonding characteristics, and reactivity. Through this comprehensive analysis, we aim to illuminate the profound influence of sp2-hybridized atoms on the biological activity of this intriguing molecule.
Identification of sp2-Hybridized Atoms: Select The Atoms In Histrionicotoxin 283a That Are Sp2-hybridized.
Histrionicotoxin 283a is a polycyclic alkaloid with a complex molecular structure. The molecule contains several sp2-hybridized carbon atoms, which play a crucial role in its overall structure and function.
Sp2 hybridization occurs when a carbon atom forms three sigma bonds and one pi bond. The three sigma bonds are formed by the overlap of the sp2 hybrid orbitals with the orbitals of other atoms, while the pi bond is formed by the overlap of the unhybridized p orbital with the p orbital of another atom.
Carbon Atoms in Aromatic Rings, Select the atoms in histrionicotoxin 283a that are sp2-hybridized.
The carbon atoms that are part of the aromatic rings in histrionicotoxin 283a are sp2-hybridized. This is because the aromatic rings are composed of alternating single and double bonds, which require the carbon atoms to have a trigonal planar geometry.
| Carbon Atom | Position | Hybridization State |
|---|---|---|
| C1 | Ring A | sp2 |
| C2 | Ring A | sp2 |
| C3 | Ring A | sp2 |
| C4 | Ring A | sp2 |
| C5 | Ring A | sp2 |
| C6 | Ring A | sp2 |
| C7 | Ring B | sp2 |
| C8 | Ring B | sp2 |
| C9 | Ring B | sp2 |
| C10 | Ring B | sp2 |
| C11 | Ring B | sp2 |
| C12 | Ring B | sp2 |
Answers to Common Questions
What is the significance of sp2-hybridization in histrionicotoxin 283a?
Sp2-hybridization plays a crucial role in determining the geometry and bonding characteristics of histrionicotoxin 283a. It contributes to the molecule’s stability, reactivity, and ability to interact with other molecules.
How do sp2-hybridized carbon atoms contribute to the biological activity of histrionicotoxin 283a?
The sp2-hybridized carbon atoms form part of the aromatic rings in histrionicotoxin 283a, which are essential for its biological activity. These rings provide a rigid and planar structure that facilitates specific interactions with target molecules.